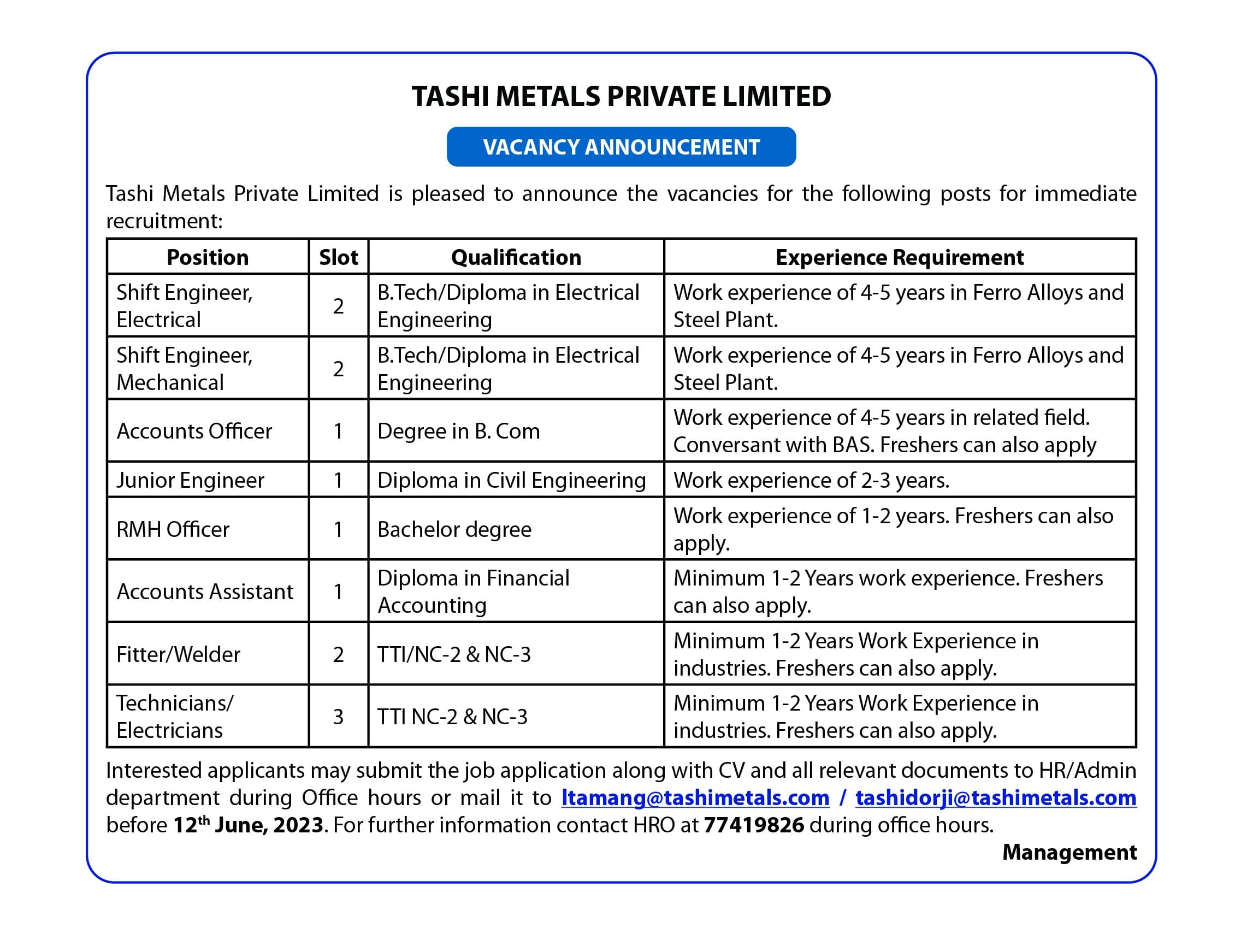
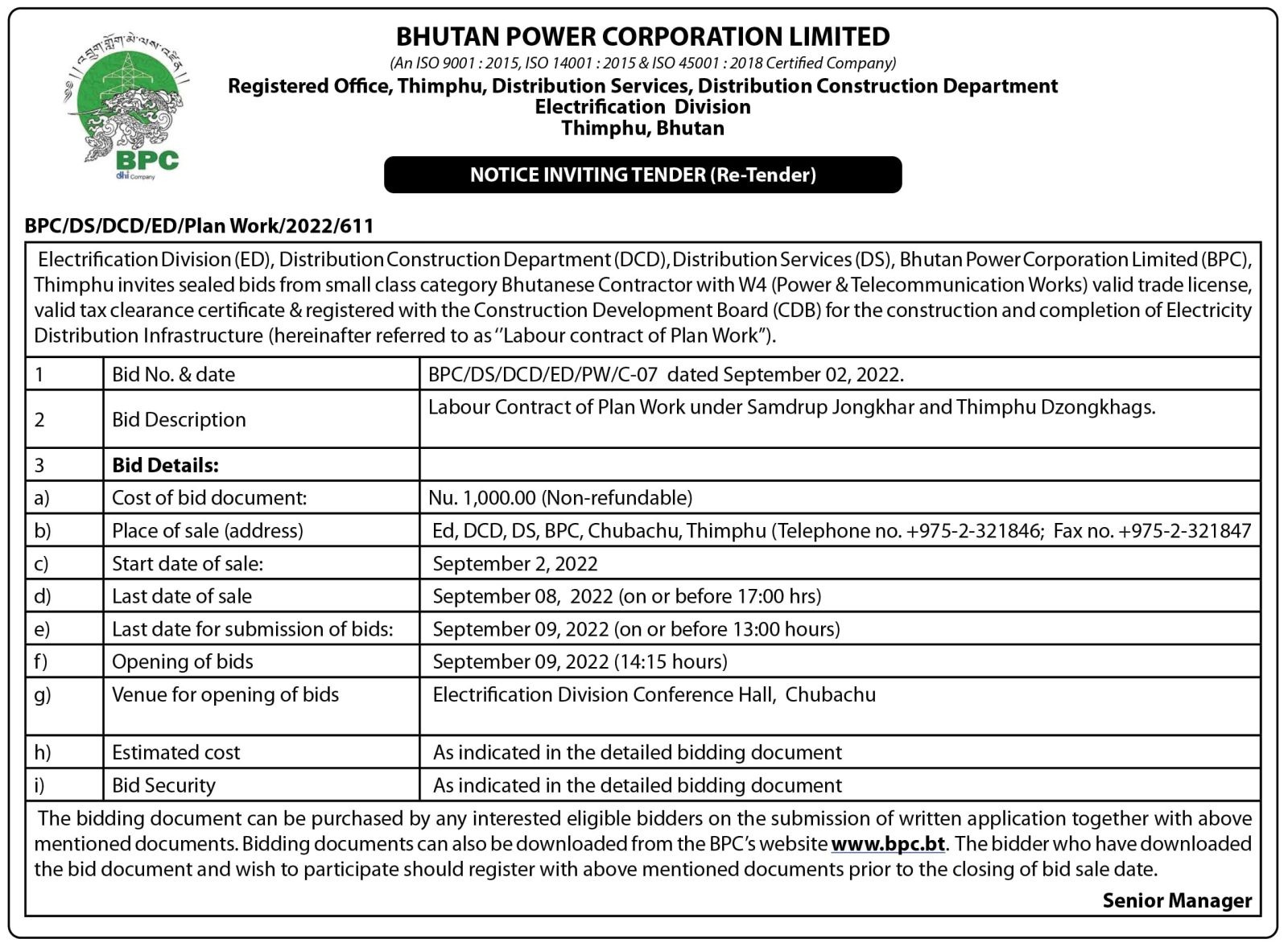
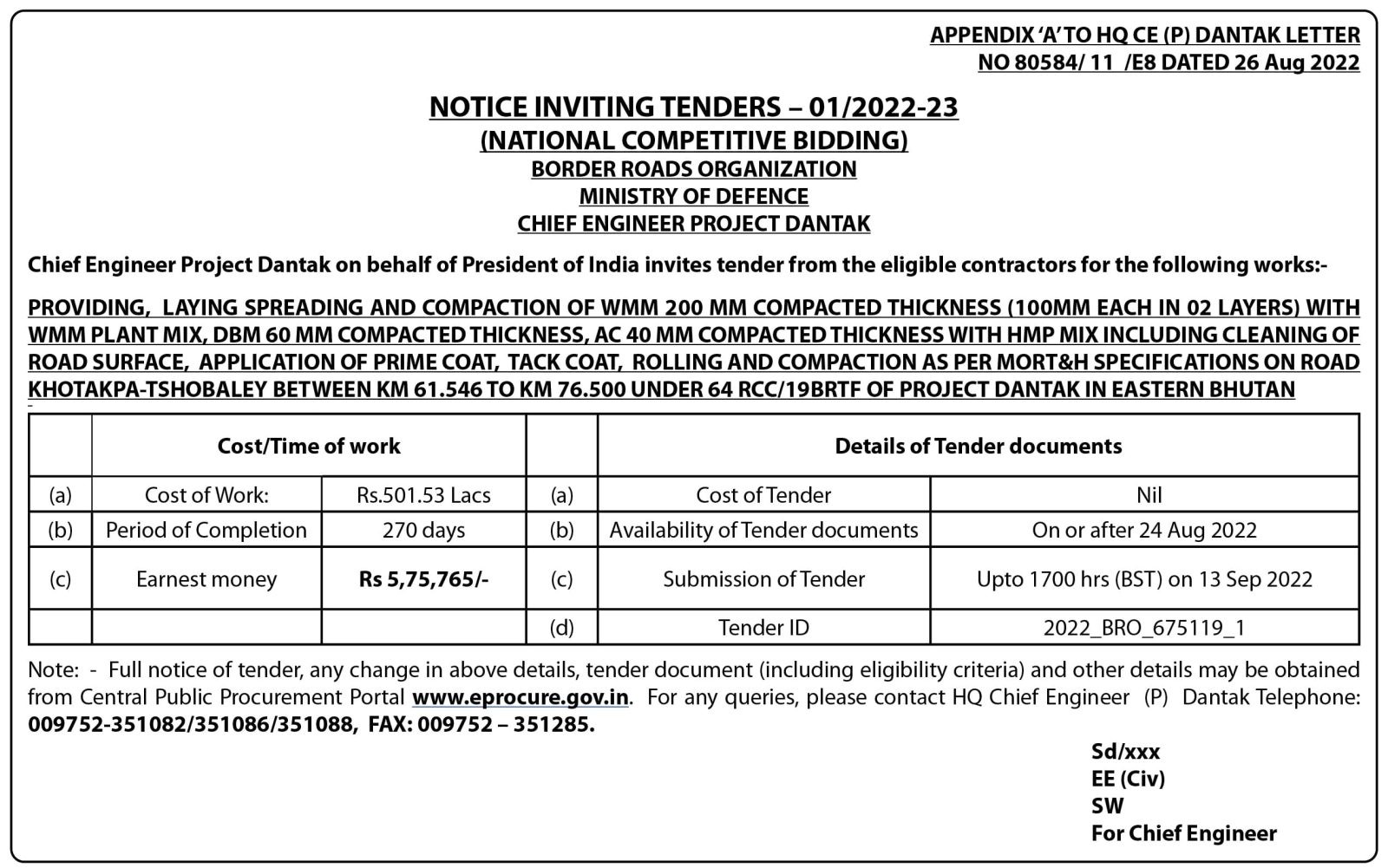
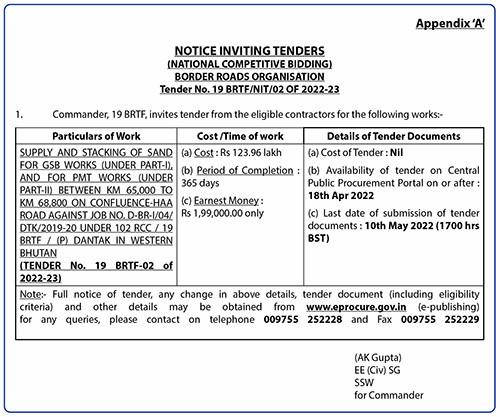
The FDA US authorized BioNtec Pfizer and Moderna vaccines for children aged six months to five years on June 17
The Ministry of Health (MoH) is reviewing other countries that have rolled out Covid-19 vaccines for children aged six months to five years, following the news from the Food and Drug Administration (FDA) that approved the Pfizer and Moderna vaccines for children under these age range.
For this, the discussion is in the process of whether or not to vaccinate the children in the country.
Program Officer of the health ministry, Tashi Dawa said that the ministry is still monitoring other countries that have vaccinated children from 6 months to 4 years and have not made any decision yet.
“We are still in the process of discussing whether to vaccinate the children,” he said, adding that parents of unvaccinated children between the ages of six months and four years have widespread worries about the immunizations’ safety and possible negative effects.
However, the U.S. Food and Drug Administration approved the Pfizer-BioNTech and Moderna COVID-19 vaccines for emergency use. On June 17, the COVID-19 vaccine for the disease will be approved for use in infants as young as six months.
The only thing stopping them at this time, Tashi Dawa, is that many parents in the US have not vaccinated their children in that age range and many are reluctant.
“Everything is ready from the ministry’s side and they have also decided to pre-order 84,000 doses of vaccines for children aged six months to five years,” he said adding the ministry asserts that they will wait to decide to observe how well the immunizations perform in children who have received them in other countries.
According to health officials, many people have been concerned about this age group of kids because they are the only ones who have not received the vaccination.
“We also directly cannot vaccinate our kids as soon as the FDA approves, we have to see and observe the side effects, and then only decide,” he said adding that the main worry is that many parents have shown reluctance to vaccinate their children in other countries.
Meanwhile, the parents of children under five in the country are ecstatic that the FDA in the US has approved vaccines for children, and many say they intend to vaccinate their children as soon as the MoH makes an announcement.
Some parents shared that she has been in constant fear that if their child contracts covid-19. “We are afraid if our child gets covid because they are not vaccinated,” they said adding that though there are not many cases of the virus in the country, covid is not ended still.
However, the children aged five to eleven years will get their first booster dose by this September.
Tshering Pelden from Thimphu